Lewis structure dot draw formaldehyde
Table of Contents
Table of Contents
Are you struggling to draw Lewis dot structures? Do those little dots and lines confuse you? Fear not! In this article, we’ll break down exactly how to draw Lewis dot structures step by step, so you can finally conquer this essential skill in chemistry.
When it comes to drawing Lewis dot structures, there can be a lot of confusion and frustration. Students often struggle with knowing where to put the dots and how many to use. They may also have trouble determining the central atom and how to arrange the atoms in the structure. Additionally, there can be confusion regarding how to handle molecules with multiple bonds or lone pairs.
The first step in drawing a Lewis dot structure is to determine the total number of valence electrons in the molecule. This can be done by adding up the valence electrons of all the atoms involved. The valence electrons are the electrons in the outermost shell of an atom. Once you have the total number of valence electrons, you can start placing them around the atoms in the structure.
In summary, to draw a Lewis dot structure, you need to: determine the total number of valence electrons, identify the central atom, arrange the atoms in the structure, place the valence electrons around the atoms, and ensure that each atom has a full octet (except for hydrogen).
Step by Step Guide to Drawing Lewis Dot Structures
When I was first learning how to draw Lewis dot structures, I found it helpful to follow a step by step guide. Here is the method I use:
1. Determine the total number of valence electrons in the molecule.
2. Identify the central atom (usually the least electronegative element) and draw it in the center of the structure. Connect the other atoms to the central atom using single bonds.
3. Place the remaining valence electrons around the atoms in the structure, starting with the outer atoms.
4. Ensure that each atom (except for hydrogen) has a full octet by adding double or triple bonds where necessary.
5. Check the structure to make sure that all the valence electrons have been used up and each atom has a full octet.
Mistakes to Avoid When Drawing Lewis Dot Structures
One common mistake students make when drawing Lewis dot structures is failing to identify the central atom. Remember, the central atom is usually the least electronegative element and is connected to the other atoms in the structure.
Another mistake to avoid is using too many or too few valence electrons. Be sure to double check your calculations to ensure that you are using the correct number of valence electrons for each atom.
Practice Makes Perfect
Drawing Lewis dot structures can be a complex and challenging task, but with practice, it will become easier. Keep practicing and don’t be afraid to ask for help when you need it.
Tips for Drawing Lewis Dot Structures
Here are some additional tips to help you master Lewis dot structures:
- Start with the simpler structures and work your way up to the more complex ones.
- Keep track of your valence electrons by drawing a tally mark for each one.
- Remember that lone pairs take up space and can affect the overall shape of the molecule.
Question and Answer
Q: How do I know which atom to place in the center of the Lewis dot structure?
A: The central atom is usually the least electronegative element. If there are multiple choices for the central atom, choose the one that can form the most bonds.
Q: Can hydrogen have more than two valence electrons?
A: Hydrogen can only have two valence electrons. It can never have more than that.
Q: How do I know when to use double or triple bonds in the Lewis dot structure?
A: Double or triple bonds are used when an atom does not have a full octet. Typically, this occurs with atoms such as nitrogen, oxygen, and sulfur.
Q: What is the purpose of drawing Lewis dot structures?
A: Drawing Lewis dot structures helps us understand the bonding between atoms in a molecule and predict the shape of the molecule.
Conclusion of How to Draw Lewis Dot Structures
Drawing Lewis dot structures is an essential skill in chemistry. By following these step by step instructions and avoiding common mistakes, you’ll be well on your way to mastering the art of drawing Lewis dot structures.
Gallery
How To Draw A Lewis Structure

Photo Credit by: bing.com / lewis structure dot draw formaldehyde
Chemistry Notes

Photo Credit by: bing.com /
Lewis Structure | Chemistry Classroom, Teaching Chemistry, Chemistry

Photo Credit by: bing.com / lewis structure dot draw chemistry molecular structures infographic notes worksheet organic classroom help simple geometry drawing diagram electron worksheets babe
3 Ways To Draw Lewis Dot Structures - WikiHow

Photo Credit by: bing.com / lewis dot structures draw formel wikihow ways
How To Draw Lewis Dot Structure
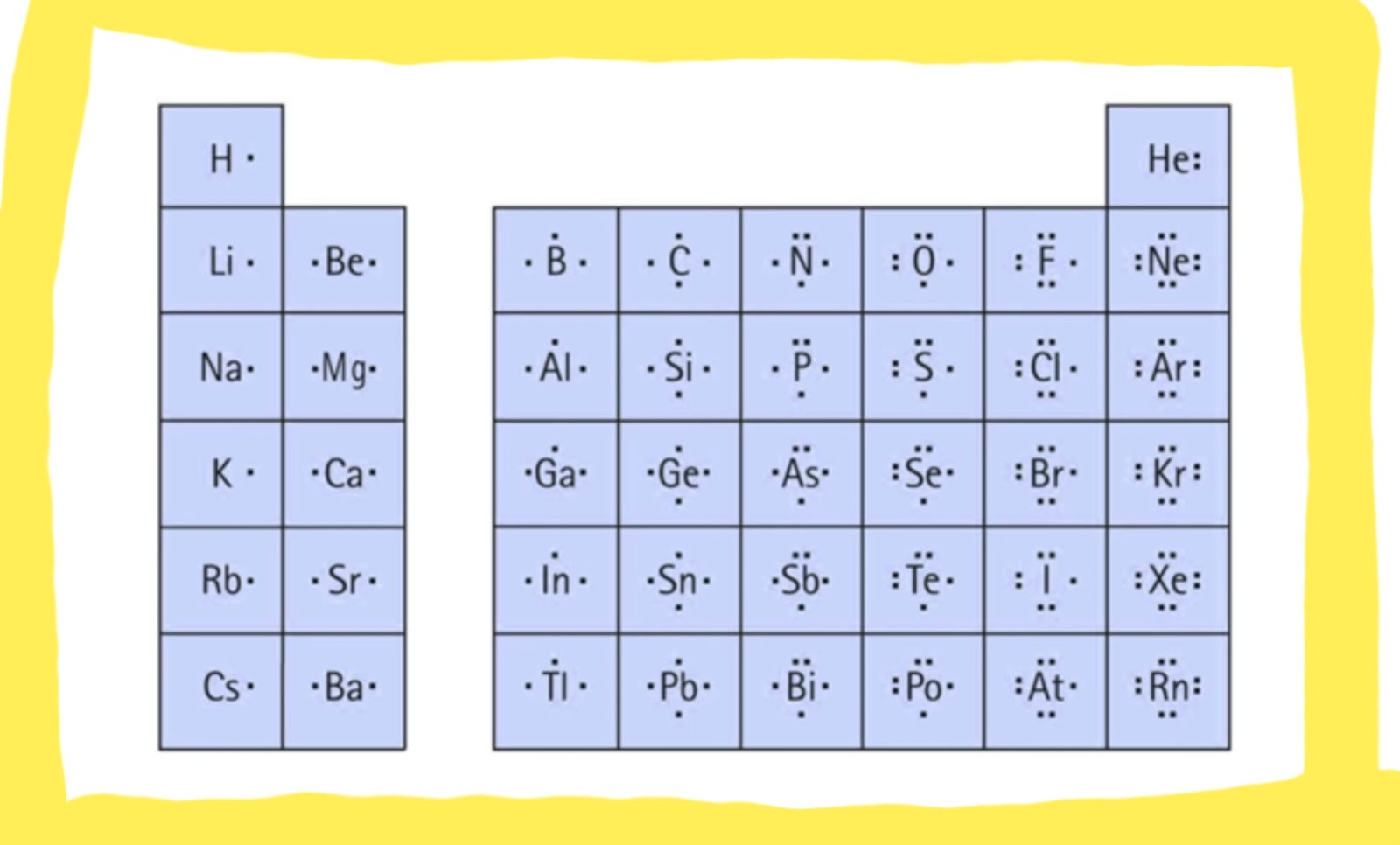
Photo Credit by: bing.com / lewis dot structure structures diagram valence draw electrons drawing represent often used number